MitoZoa Search
The MitoZoa database can be queried using the "General Search" menu alone or in combination with one of the following specialized menus:1) Gene Order
2) Non-Coding Region (NCR)
3) Gene Content
Single entries are shown in the MZ format and can be also displayed/downloaded in EMBL-like and FASTA formats.
Entry lists can be downloaded in a tab-separated simple text format (.txt) or in a table format (.xls), and report different data depending on the search. The data included in these files consist at least of the following fields: Accession number, Definition, Taxonomy, Organism, Genome Length, and Molecule Topology.
The type and format of retrievable outputs for each search menu is reported below:
| Menu | Retrieved data | Format |
|---|---|---|
| General Search | Entry list Single entry |
tab-separated simple text (.txt) table (.xls) EMBL, FASTA |
| Gene Order | Entry list Gene order |
tab-separated simple text (.txt) table (.xls) FASTA-like |
| NCR | Entry list NCR sequences |
tab-separated simple text (.txt) table (.xls) FASTA |
| Gene Content | Entry list Gene sequences |
tab-separated simple text (.txt) table (.xls) FASTA |
General Search Menu
The "General Search Menu" allows text searches in all or in a single field of the MitoZoa entries, and is intended to provide information on general mtDNA features.In addition to standard fields of the EMBL format (such as AC number, Definition, Keywords, References, Organism Species, and Organism Classification), four MitoZoa-specific fields can be searched by this menu:
- Genetic Code
- Congeneric Species
- MitoZoa Reannotation Summary
- Base Composition.
- Accession Number
This field reports only the EMBL/Genbank/DDBJ or the RefSeq accession (AC) number whose original annotation was modified by the MitoZoa reannotation pipeline. An AC number absent in this field may be searched in the "MitoZoa Reannotation Summary" (where both the EMBL and the possible RefSeq AC numbers referred to the same sequence are cited). However, we want to stress that the query of MitoZoa by "Organism Name" or "Taxonomy" fields is the best way to get exhaustive results.
- Genetic Code
This field allows the selection of mtDNA entries using a specific genetic code.
A drop-down menu reports the list of used metazoan Genetic Codes (Table 1), including:
- five mt translation tables compiled by the NCBI's staff, numbered and named as in the NCBI web page;
- three extra tables (5mod, 5bis, and 9mod) corresponding to three genetic codes not yet described by the NCBI staff.
Table 1. Translation tables describing the mitochondrial genetic codes used by Metazoa, according to the NCBI Genetic Code web page
| Name a | Number | Source |
|---|---|---|
| Vertebrate mt code | 2 | NCBI |
| Mold, Protozoan, and Coelenterate mt code and the Mycoplasma/Spiroplasma code | 4 | NCBI |
| Invertebrate mt code | 5 | NCBI |
| Echinoderm and Flatworm mt code | 9 | NCBI |
| Ascidian mt code | 13 | NCBI |
| Table 5 with modified ATA meaning (Met->Ile) | 5mod | New b |
| Table 5 with modified TAA meaning (Stop->Tyr) | 5bis | New c |
| Table 9 with modified AAA meaning (Asn->Lys) | 9mod | New d |
b: new translation table used by the two Porifera Hexactinellida, Iphiteon panicea (EF537576) and Sympagella nux (EF537577). This genetic code corresponds to translation table 5, except for the translation of ATA codon to Ile instead of Met (Haen et al. 2007).
c: new translation table used in the nematode Radopholus similis (FN313571). This genetic code corresponds to translation table 5, except for the translation of TAA codon to Tyr instead of stop codon (Jacob et al 2009).
d: new translation table used by the Hemichordata Saccoglossus kowalevskii (AY336131). This genetic code corresponds to translation table 9, except for the translation of AAA codon to Lys instead of Asn (Smith, Beckenbach, and Scouras, unpublished).
- Congeneric Species
This check box allows the selection of all congeneric mtDNA entries belonging to a given taxon, which has to be defined by filling the "Taxonomy" text box. When the taxon is not defined (i.e. the "Taxonomy" box is left empty), the result consists of all congeneric species found in Metazoa.
- MitoZoa Reannotation Summary
All corrections and improvements of the original mtDNA entries, made through the MitoZoa semi-automatic reannotation pipeline, are summarized in the "MitoZoa Reannotation Summary" (MRS), which is shown in red in the MZ format and is included in the Comment field of the EMBL-like format.
The first standardized message of MRS indicates the entry AC number whose annotation was modified by MitoZoa: it can be a EMBL/Genbank/DDBJ or a RefSeq entry, being the latter marked by the presence of an underscore in the AC number. Other AC numbers derived from (i.e. RefSeq) or giving rise to (i.e. EMBL/Genbank/DDBJ) the above-mentioned reannotated ENTRY are possibly reported in the second standardized message (Figure 1).
The remaining portion of the "MitoZoa Reannotation Summary" (Figure 1) is divided into three sections, indicated by capitalized titles:
1) GENERAL REANNOTATION, which lists modifications concerning all EMBL fields, except the Feature Table (FT);
2) FEATURE TABLE REANNOTATION, which lists all modifications concerning the FT;
3) ADDITIONAL INFORMATION, which lists genomic information absent or not clearly reported in the original entry.
In each section, the type and source of entry corrections/improvements are described using standardized messages (Figure 1). Moreover, the "Modification Codes" (Mcode, Table 2) indicate the inference on which the modification was based or describe the type of modification. The Mcodes are always reported in square brackets. Table 3 reports the standardized messages used in the "MitoZoa Reannotation summary", and those associated to the corresponding modified FT lines.
Table 2. Modification codes (Mcodes) reported in the "MitoZoa Reannotation Summary" or associated to FTqualifier
| MCode | Meaning | Notes |
|---|---|---|
| IC | Inferred by Curator | Used for obvious erroneous annotations; mtDNA topology; limits of "genes" different from that of "CDS" for protein-encoding genes without introns; etc |
| IR | Inferred by Reference | The reference is indicated only if different from that of the original entry, using the message "see PUBMED: number". |
| IPA | Inferred by Program Analysis | Inferred by Blast, PatSearch, Arwen, or tRNAscanSE programs (Altschul et al. 1990; Lowe and Eddy 1997; Pesole, Liuni, and D'Souza 2000; Laslett and Canback 2008) |
| IMA | Inferred by Multi-alignment Analyses | Inferred by multi-alignment of protein-coding genes |
| NV | Not Validated | Feature not validated by the MitoZoa reannotation pipeline: it is followed by a more specific Mcode |
| AI | Annotation Improvement | Additional information improving the mtDNA annotation: it is followed by a more specific Mcode |
| SA | Sporadic Analysis | Sporadic detailed analyses of protein-coding, tRNA and rRNA genes |
Figure 1. Structure of the "MitoZoa Reannotation Summary", including the list of standardized messages. Gray background indicates the most useful standardized messages that can facilitate specific searches in the "MitoZoa Reannotation Summary" field.
The meaning of some standardized messages is explained within square brackets. The type of data is reported within < >. Alternative messages are separated by "or".
|
The genome annotation was derived from <INSDCa or RefSeq AC number>.
The sequence is also reported in <RefSeq or INSDC AC number>. [AC number derived from or giving rise to the reannotated mtDNA entry indicated in the previous line] GENERAL REANNOTATION - modification of <field>: <standardized message> [Mcode]; [general structure of the standardized messages within this section] - modification of the ID field: error in molecule topology [Mcode]; - modification of the DE field: partial genome, missing at least part of the control region or missing at least part of <gene:limits> [Mcode]; - modification of the DE field: improvement of the description information [Mcode]; - modification of the DE field: information on <free text> restored from EMBL [Mcode]; - modification of the DE field: added information on the gender type [Mcode]; - modification of the OS field: <free text> [Mcode]; - modification of the OC field: changes in the NCBI Taxonomy DB [Mcode]; FEATURE TABLE REANNOTATION - error in strand of <gene: corrected limits> [Mcode]; - elimination of the erroneous < FTkey:limits> [Mcode]; [only for FTkey undoubtedly erroneous] - unannotated <gene> at position <limits>; - modified boundaries of <gene:new limits> (old: <old limits>); - <gene:limits > erroneously annotated in the original entry as <old gene name> [Mcode]; - anticodon specificity modified from <old gene name> to <new gene name:limits> [Mcode]; [only for synonymous codons recognized by two tRNAs: anticodon specificity not reported or erroneous] - trnM(CAU) <limits> specified as <initiator or elongator> [Mcode]; - <gene:limits> not validated [Mcode]; [gene not validated by the MitoZoa reannotation pipeline] - <gene:limits> not validated due to short size [Mcode]; [tRNA genes with size lower than 45 bp were not checked by the MitoZoa tRNA reannotation pipeline due to inability of these genes to form a complete secondary structure] - loss of highly conserved aminoacidic region(s) in <gene:limits> can be recovered by frameshift(s) (limits of the new ORF with frameshift: <corrected limits>) [Mcode]; - loss of highly conserved aminoacidic region(s) in <gene:limits> can be recovered by nt substitution(s) (limits of the new ORF: <corrected limits>) [Mcode]; ADDITIONAL INFORMATION - partial genome: unsequenced gap(s) [Mcode]; [presence of gap FTkey] - partial genome: missing at least part of the control region or missing at least part of <gene:limits> [Mcode]; [partial genome, as defined by the presence of partial genes in the entry or by reference] - complete subgenomic circle [Mcode]; - gender-specific mtDNA due to DUI (doubly uniparental inheritance) [Mcode]; - tRNA editing: <free text (see PUBMED:ID number)> [Mcode]; - tRNA editing in <gene:limits (see PUBMED:ID number)> [Mcode]; - frameshift due to ribosomal slippage or RNA editing in <gene:limits> [Mcode]; - uncertain frameshift due to ribosomal slippage or RNA editing in <gene:limits>: MitoZoa Curator analyses suggest low support [Mcode]; - some/all tRNAs might lack a well-paired aminoacyl arm or the 3' portion of the aminoacyl stem <(see PUBMED:ID number)> [Mcode]; - many short tRNAs lacking the D- or T-arm <(see PUBMED:ID number)> [Mcode]; - all tRNAs validated by reference [Mcode]; - all tRNAs validated thanks to data kindly provided by <Authors> [Mcode]; - list of pseudo: <gene1:limits, gene2:limits, FTkey:limits> [Mcode]; - putative four-base codon of cox1 was not experimentally validated as start codon (see PUBMED:ID number) [Mcode]; - all tRNA genes are contained in the unsequenced gaps [Mcode]; - rRNA and tRNA not annotated but presence of several long NCRs [Mcode]; - MitoZoa Curators annotated all genes [Mcode]; - presence of <group I or group II> intron in <gene:limits> [Mcode]; - unidentified control region [Mcode]; [only for vertebrates: the control region was not-sequenced or not-annotated] - unidentified <gene list> [Mcode]; [genes not-sequenced or unannotated] - <gene> is not mitochondrially encoded [Mcode]; [only for usually mt-encoded genes, whose absence in the mtDNA was confirmed by reference check] - <gene> is mitochondrially encoded [Mcode]; [only genes not usually mt-encoded] - <3' or 5'> end of <rRNA gene:limits> not adjacent to the limit of flanking gene [Mcode]; - both ends of <rRNA gene:limits> not adjacent to the limits of flanking genes [Mcode]; |
a: INSDC indicates entries of the International Nucleotide Sequence Database Collaboration, comprising DDBJ, EMBL and GenBank primary databases.
Table 3. Standardized messages included in the "MitoZoa Reannotation Summary" and in FT lines, together with the possible gene categories involved in each message type. Messages are listed according to the Section of the "MitoZoa Reannotation Summary" containing them.
Alternative messages are separated by "or". The type of data or alternative messages are reported within < >. CDS: protein-coding genes. AnC: anticodon sequence.
| "MitoZoa Reannotation Summary" message | FTqualifier "/note" | FTqualifier "/inference" a | Gene | Mcode b |
|---|---|---|---|---|
| none | putative NCR limits due to uncertain rRNA boundaries | NCR | IC | |
| none | putative NCR 1 bp-long flanking rRNA | NCR | IC | |
| none | NCR inside intron | NCR | IC | |
| none | longest non-coding region | NCR | IC | |
| none | contains a gap feature | NCR | IC | |
| none | high sequence similarity to <tRNA>; unusual anticodon: <AnC> | profile:Arwen:1.2; alignment:<muscle or mafft> | tRNA | AI:IPA, AI:IR, IC:SA |
| none | <editing event;> unusual anticodon: <AnC>; | profile:Arwen:1.2 | tRNA | AI:IPA, AI:IR |
| none | validated partial cloverleaf structure | profile:Arwen:1.2 | tRNA | IPA |
| none | elimination of the partial symbol(s) | All | IC, IR | |
| none | validated by reference | All | IC, IR | |
| none | validated by blastn analysis | alignment:blastn | All | IPA, IC |
| none | <5' or 3'> part of split ribosomal RNA | rRNA | IC | |
| In "Feature Table Reannotation" section | ||||
| "MitoZoa Reannotation Summary" message | FTqualifier "/note" | FTqualifier "/inference" | Gene | Mcode b |
| error in strand of <gene:corrected limits> | error in strand annotation | nucleotide motif:PatSearch:2.0; profile:<tRNAscanSE:1.23 or Arwen:1.2>; alignment:<muscle or mafft> |
tRNA | IPA, IR, IC:SA |
| error in strand of <gene:corrected limits> | error in strand annotation | alignment:<blastn or muscle or mafft> | rRNA, CDS | IPA, IR, IMA |
| elimination of the erroneous <gene:limits> | All | IC, IR | ||
| unannotated <gene> at position <limits> | added by MitoZoa Curator | All | IC, IR | |
| modified boundaries of <gene:new limits> (old: <old limits>) | modified gene boundaries <and anticodon specificity> | profile:<tRNAscanSE:1.23 or Arwen:1.2>; alignment:<muscle or mafft> | All | IPA, IR, IC:SA, IMA |
| <gene:limits> erroneously annotated in the original entry as <old gene name> | error in gene name | alignment:<blastn or muscle or mafft> | rRNA, CDS | IPA, IR, IMA |
| <gene:limits> erroneously annotated in the original entry as <old gene name> | error in gene name | profile:Arwen:1.2; nucleotide motif:PatSearch:2.0; alignment:<muscle or mafft> | tRNA | IPA, IR, IC:SA |
| anticodon specificity modified from <old gene name> to <new gene name:limits> | modified anticodon specificity | nucleotide motif:PatSearch:2.0; profile:<tRNAscanSE:1.23 or Arwen:1.2> |
tRNA | IPA |
| trnM(CAU):<limits> specified as <initiator or elongator> | specified as <initiator or elongator> trnM(CAU), based on signals present in primary and secondary structures | profile:Arwen:1.2 | tRNA | IPA |
| <gene:limits> not validated due to <short or long>size | not validated due to <short or long> size | tRNA | NV:IC | |
| <gene:limits> not validated | not validated by PatSearch and Arwen check | tRNA | NV:IPA | |
| <gene:limits> not validated due to ambiguous bases in anticodon arm | not validated due to positions with ambiguous bases in anticodon arm | tRNA | NV:IPA | |
| <gene:limits> not validated due to the presence of N-stretch | not validated due to the presence of N-stretch | tRNA | NV:IPA | |
| loss of highly conserved aminoacidic region(s) in <gene:limits> can be recovered by frameshift(s) (limits of the new ORF with frameshift: <corrected limits>) | loss of highly conserved aminoacidic region(s) can be recovered by frameshift(s) | alignment:<muscle or mafft> | CDS | IMA |
| loss of highly conserved aminoacidic region(s) in <gene:limits> can be recovered by nt substitution(s) (limits of the new ORF: <corrected limits>) | loss of highly conserved aminoacidic region(s) can be recovered by nt substitution(s) | alignment:<muscle or mafft> | CDS | IMA |
| In "Additional Information" section | ||||
| "MitoZoa Reannotation Summary" message | FTqualifier "/note" | FTqualifier "/inference" | Gene | Mcode b |
| 5'end of <rrn:limits> not adjacent to the limit of flanking gene | 5'end not adjacent to the limit of flanking gene | rRNA | AI:IC | |
| 3'end of <rrn:limits> not adjacent to the limit of flanking gene | 3'end not adjacent to the limit of flanking gene | rRNA | AI:IC | |
| both <rrn:limits> ends not adjacent to the limits of flanking genes | both ends not adjacent to the limits of flanking genes | rRNA | AI:IC | |
| <gene_1:limits> contains the entire <gene_2:limits>, which is encoded on the <complementary or same> strand | contains the entire <gene_2:limits>, which is encoded on the <complementary or same>strand | All | AI:IC, AI:IR | |
| " | <entirely> contained in <gene_1:limits>, which is encoded on the <complementary or same> strand | All | AI:IC, AI:IR | list of pseudo:<gene_1:limits, FTkey:limits> | pseudo | All | AI:IC, AI:IR |
b: as reported in Table 2.
- Base Composition
This sub-menu allows the selection of all mtDNA entries having a specific base composition.
Entries can be selected based on min, max, and range of the following parameters, calculated on the whole mtDNA sequence and on the strand stored in the database:
- percentage of single bases (where "N" indicates any base different from A,C,G, T),
- GC percentage,
- AT_skew and GC_skew.
The GC_skew and AT_skews indicate compositional differences between the two DNA strands, and were calculated according to the formulae by Perna and Kocher (1995):
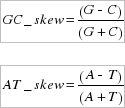
where C, G, A, and T are the occurrences of the four bases on the whole mtDNA sequence and on the strand stored in the database. Skew values range from -1 to +1, with a value equal to zero corresponding to the absence of compositional strand asymmetry.
Gene Order Menu
The gene order is reported as a string of standardized gene names (Table 4), with a "-" sign preceding genes encoded by the minus strand. Genes interrupted by introns or splitted in two parts are indicated with the standardized gene name followed by "_5" or "_3" for the 5'- and 3'-end, respectively. Pseudogenes are indicated with the prefix "psi_" before the standardized gene name.For complete circular mtDNAs, the first gene of the string is always nad1; for partial and linear mtDNAs, the first gene of the string is the first gene annotated in the Feature Table of the entry.
The gene order is reported in a FASTA-like format (Figure 2) that can be downloaded and directly used as input in programs analysing gene order such as CREx (Bernt et al. 2007). Each gene order string given in the FASTA-like format is associated to a header reporting:
1) The accession number of the entry;
2) A token specifying genome topology and status, with P: partial, L: linear, and PL: partial plus linear;
3) the organism name.
- "Search for Gene Order"
This sub-menu enables the retrieval of all mtDNA entries having a given string of two or more genes. Each gene string is searched in both the forward and reverse/complement orientation (i.e. the string "nad4L nad4 -nad6" is searched also as "nad6 -nad4 -nad4L"), as these orientations are biologically equal and depend only on the strand stored in the database.
The gene string can be directly written into the relevant box using the standardized gene names (Table 4), or it can be generated via the "Add gene name" button. This tool allows user to consecutively select the genes of interest from a drop-down list, and to automatically write them in the relevant text box.
- "Gene order as in"
This sub-menu allows retrieving:
1) all mtDNA entries whose gene order is equal to that of a given species, selected using a drop-down list;
2) all gene orders present in a given taxonomic group, whose name has to be written in the "Genus or higher taxonomic rank" text box.
- "Dataset in the gene order file"
This sub-menu allows selecting the gene order output among one of the following datasets, containing:
1- All genes
2- No tRNA (i.e. all genes excluding tRNAs)
3- Only CDS (i.e. only protein genes)
The check box "Show notes on non-validated genes" permits to download gene order dataset(s) containing the flag "[NV]" (Not Validated) after each gene that was not-validated by the MitoZoa pipeline, and thus has uncertain annotation. This option helps user to identify the most controversial points of mtDNA annotation and gene order.
Figure 2. Example of FASTA-like format reporting the gene order of four MitoZoa entries
| >J01415|Homo sapiens nad1 trnI -trnQ trnM nad2 trnW -trnA -trnN -trnC -trnY cox1 -trnS(UCN) trnD cox2 trnK atp8 atp6 cox3 trnG nad3 trnR nad4L nad4 trnH trnS(AGY) trnL(CUN) nad5 -nad6 -trnE cob trnT -trnP trnF rrnS trnV rrnL trnL(UUR) >NC_007175|Crassostrea virginica nad1 nad4L trnW cox1 rrnL_3 cox3 trnI trnT trnE cob cox2 trnS(AGN) trnL(UUR) trnP trnG rrnS trnM trnK trnC trnV trnD rrnL_5 trnM trnS(UCN) trnY atp6 nad2 trnR trnH nad4 trnN nad5 nad6 trnQ nad3 trnL(CUN) trnF trnA >AB055624|P|Inversidens japanensis sex male haplo. male type cox3 atp6 atp8 trnD nad4L nad4 -nad6 -trnG -nad1 -trnL(UUR) -trnV -trnI -trnC -trnQ nad5 -trnF -cob -trnP -trnN -trnL(CUN) -rrnL -trnY -trnT -trnK -rrnS -trnR -trnW -trnM -nad2 -trnE -trnS(AGN) -trnS(UCN) -trnA nad3 cox2 trnH cox1 >BN001179|PL|Hydra vulgaris chromosome 1 -psi_cox1 trnM rrnL trnW cox2 atp8 atp6 cox3 nad2 nad5 psi_cox1 |
Table 4. Standardized names and NCR codes for the mt genes of Metazoa
| Product 1 | Gene Code | NCR code 2 |
|---|---|---|
| ATP synthase subunit 6 | atp6 | A6 |
| ATP synthase subunit 8 | atp8 | A8 |
| ATP synthase subunit 9 | atp9 | A9 |
| cytochrome b | cob | CB |
| cytochrome c oxidase subunit I | cox1 | C1 |
| cytochrome c oxidase subunit II | cox2 | C2 |
| cytochrome c oxidase subunit III | cox3 | C3 |
| NADH dehydrogenase subunit 1 | nad1 | N1 |
| NADH dehydrogenase subunit 2 | nad2 | N2 |
| NADH dehydrogenase subunit 3 | nad3 | N3 |
| NADH dehydrogenase subunit 4 | nad4 | N4 |
| NADH dehydrogenase subunit 4L | nad4L | 4L |
| NADH dehydrogenase subunit 5 | nad5 | N5 |
| NADH dehydrogenase subunit 6 | nad6 | N6 |
| Unusual protein-coding genes | ||
| Unknown or hypothetical protein 3 | orf | OR |
| Putative DNA/RNA polymerase | dnaB | DB |
| Homing endonuclease | heg | HG |
| DNA mismatch repair protein mutS | mutS | US |
| SecY-independent transporter protein 4 | mttB | MB |
| Ribosomal RNA genes | Large ribosomal subunit RNA | rrnL | RL |
| Small ribosomal subunit RNA | rrnS | RS |
| Transfer RNA genes | ||
| tRNA-Ala | trnA | TA |
| tRNA-Cys | trnC | TC |
| tRNA-Asp | trnD | TD |
| tRNA-Glu | trnE | TE |
| tRNA-Phe | trnF | TF |
| tRNA-Gly | trnG | TG |
| tRNA-Gly(AGR) | trnG(AGR) | GA |
| tRNA-Gly(GGN) | trnG(GGN) | GG |
| tRNA-His | trnH | TH |
| tRNA-Ile | trnI | TI |
| tRNA-Ile(CAU) | trnI(CAU) | IC |
| tRNA-Lys | trnK | TK |
| tRNA-Leu(CUN) | trnL(CUN) | LC |
| tRNA-Leu(UUR) | trnL(UUR) | LU |
| tRNA-Met | trnM | TM |
| tRNA-Met(UAU) | trnM(UAU) | MU |
| Elongator tRNA-Met | trnM(CAU)e | ME |
| tRNA-FormylMet | trnM(CAU)f | MF |
| tRNA-Asn | trnN | TN |
| tRNA-Pro | trnP | TP |
| tRNA-Gln | trnQ | TQ |
| tRNA-Arg | trnR | TR |
| tRNA-Arg(UCU) | trnR(UCU) | RU |
| tRNA-Ser(AGY) | trnS(AGY) | SA |
| tRNA-Ser(AGN) | trnS(AGN) | SA |
| tRNA-Ser(UCN) | trnS(UCN) | SU |
| tRNA-Thr | trnT | TT |
| tRNA-Unknown 5 | trnUk | UK |
| tRNA-Val | trnV | TV |
| tRNA-Trp | trnW | TW |
| tRNA-Tyr | trnY | TY | Pseudogenes |
| pseudo cytochrome b | psi_cob | CB |
| pseudo cytochrome c oxidase subunit 1 | psi_cox1 | C1 |
| pseudo NADH dehydrogenase subunit 1 | psi_nad1 | N1 |
| pseudo NADH dehydrogenase subunit 3 | psi_nad3 | N3 |
| pseudo NADH dehydrogenase subunit 4 | psi_nad4 | N4 |
| pseudo large ribosomal subunit RNA | psi_rrnL | RL |
| pseudo small ribosomal subunit RNA | psi_rrnS | RS |
| pseudo tRNA-Glu | psi_trnE | TE |
| pseudo tRNA-His | psi_trnH | TH |
| pseudo tRNA-Lys | psi_trnK | TK |
| pseudo tRNA-Met | psi_trnM | TM |
| pseudo tRNA-Pro | psi_trnP | TP |
| pseudo tRNA-Gln | psi_trnQ | TQ |
| pseudo tRNA-Ser(UCN) | psi_trnS(UCN) | SU |
| pseudo tRNA-Thr | psi_trnT | TT |
| pseudo tRNA-Unknown | psi_trnUk | UK |
| pseudo tRNA-Val | psi_trnV | TV |
1: The different tRNA genes for Gly, Ser and Leu are named based on the recognized codons, while the different tRNA genes for Met, Arg e Ile are named based on the anticodon sequence.
2: Abbreviations used for the definition of the Non-Coding Regions (NCR) code (see NCR section).
3: Used to indicate several non-homologous ORFs.
4: Synonymous gene names: tatC, ymf16.
5: tRNA or tRNA-like genes with an unexpected anticodon.
NCR Menu
The NCR menu allows the retrieval and download of non-coding regions (NCR) having:1) a given bp length (min, max, or range);
2) a given gene upstream and/or downstream the NCR itself. If the NCR is at the beginning or at the end of a linear or partial mtDNA, then the "mtDNA5'end" and "mtDNA3'end" codes can be used to retrieve these NCRs. The standardized gene names are reported in Table 4 and listed in a drop-down menu.
A NCR is defined as a non-coding sequence of any size located between two consecutive annotated genes: "misc_feature", "D-loop" and all other non-genic FTkeys have not been considered in the NCR boundary definition, thus, if present, they have been included in NCR sequences. Finally, all NCRs are in the plus orientation (i.e., the same orientation of the entry sequence).
Each NCR has been associated to a specific code summarizing data on species, flanking genes, and bp length of the NCR itself. This code is reported in the MitoZoa-specific FTqualifier "code", and is made up of 3 compulsory and 2 optional bits, spaced out by underscores:
1) Species bit (compulsory): 4 letters for species name. Table 5 lists the species bits for all entries;
2) Gene bit (compulsory): 2 letters for the gene preceding NCR + 2 letters for the gene following NCR (see NCR codes in Table 4). If the NCR is at the beginning or at the end of a linear or partial mtDNA, the first/last part of this bit will be "5E" (meaning: 5'-end) and "3E" (meaning: 3'-end), respectively;
3) Length bit (compulsory): the length of NCR in bp;
4) Intron bit (optional): the "ii" bit, an acronym for "inside intron", indicates NCR sequences located inside a group I or group II intron;
5) Gene copy number bit (optional): 2 letters for the copy-number of the gene upstream NCR + 2 letters for the copy-number of the gene downstream NCR. Thus, if the gene upstream (downstream) NCR is present in the mtDNA in multiple copies, then a " uj "(" dj ") bit is used, being "j" the gene copy number defined by the position in which this multi-copy gene appears in the FT, and "u" ("d") an abbreviation for "upstream" ("downstream").
As an example, a NCR of Halocynthia roretzi, located between nad6 and the second copy of trnF, having a length of 36 bp, will be named in the "code" FTqualifier as: AAGZ_N6TF_36_d2.
As further comments:
a) the longest NCR of a mtDNA entry is indicated with the standardized message "longest non-coding region" in the "note" FTqualifier;
b) NCRs contained in group I or group II introns are indicated with the standardized message "NCR inside intron" in the "note" FTqualifier;
Additional comments on NCR features are reported in Table 3.
Table 5: Species bits, organism and accession number (AC) for all entries in MitoZoa DB
Click here to Download Table 5 (xls format)
Gene Content Menu
This menu allows to obtain statistics on the mtDNA gene content, and to retrieve sub-sequences corresponding to specific genes or FTkeys.- "Gene count"
This sub-menu allows retrieving:
1) all mtDNA entries encoding for a specific number of genes, referred to the whole gene content or to a given gene category (tRNA, rRNA or CDS: protein coding genes) that can be selected using a drop-down list. This search can be also limited to only one strand;
2) all mtDNA entries where a given gene is absent, i.e. the gene is not encoded by the mtDNA, or is not present in the mtDNA entry either because of partial genome or unannotated gene. The standardized gene names are reported in Table 4 and can be combined using the "AND" Boolean operator.
3) all mtDNA entries where a gene is present in at least two copies (duplicated). The standardized gene names are reported in Table 4 and can be combined using the "AND" Boolean operator.
- "Feature retrieval"
This sub-menu allows retrieving:
1) all sequences of a specific gene, whose standardized name can be selected using a drop-down list;
2) all sub-sequences belonging to a specific FTkey category, also including the MitoZoa-specific FTkeys "NCR" and "prec_ORF". These FTkeys can be selected using a drop-down list.
Both searches can be restricted setting a limit for the length (in bp) or selecting the strand of the desired gene/FTkey. If the length text boxes are not filled, genes/FTkeys of any length are shown.
MitoZoa BLAST
The BLAST service allows sequence similarity searches by BlastN, BlastX or BlastP, against several datasets of functionally homogeneous sequences contained in MitoZoa.
| Database | Contents |
|---|---|
| mtDNA | Full sequence of all MZ entries |
| CDS_nt | All MitoZoa CDS FTkeys, with the corresponding standardized gene name |
| tRNA | All MitoZoa tRNA FTkeys, with the corresponding standardized gene name |
| rRNA | All MitoZoa rRNA FTkeys, with the corresponding standardized gene name |
| NCR≥25nt | MitoZoa Non-Coding Region FTkeys of length ≥ 25 nt, with the corresponding specific NCR code (see NCR Menu Help) |
| Protein | Translation of all CDS FTkey, excluding pseudogenes |
Taxonomy
The search will be restricted to the MitoZoa sequences that belong to the taxon selected, as reported in the "Taxonomy = Organism classification" field (examples: Arthropoda, Tunicata or Canis).
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403-410.
Bernt M, Merkle D, Ramsch K, Fritzsch G, Perseke M, Bernhard D, Schlegel M, Stadler PF, Middendorf M. 2007. CREx: inferring genomic rearrangements based on common intervals. Bioinformatics. 23:2957-2958.
Haen KM, Lang BF, Pomponi SA, Lavrov DV. 2007. Glass sponges and bilaterian animals share derived mitochondrial genomic features: a common ancestry or parallel evolution? Mol Biol Evol. 24:1518-1527.
Jacob JE, Vanholme B, Van Leeuwen T, Gheysen G. 2009 A unique genetic code change in the mitochondrial genome of the parasitic nematode Radopholus similis. BMC Res Notes. 24;2:192.
Laslett D, Canback B. 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24:172-175.
Lowe TM, Eddy SR. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25:955-964.
Perna NT, Kocher TD. 1995. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 41:353-358.
Pesole G, Liuni S, D'Souza M. 2000. PatSearch: a pattern matcher software that finds functional elements in nucleotide and protein sequences and assesses their statistical significance. Bioinformatics 16:439-450.



